- S │
- M │
- L │

Information for the public and patients
What is a clinical trial
It is necessary for patients to cooperate until we can use a new therapy for certain diseases to verify the safety and efficacy of the new therapy for a target disease. Therefore, after we first conduct a study in animals (nonclinical test), we advance to a study in humans. A study like this in humans is called a “clinical study”, and it is therapeutic research that is only performed in patients who agree to participate in it. When such research demonstrates the safety and efficacy of a new therapy, it will be of great help in treating the same disease as yours in the future.
What is critical limb ischemia
Critical limb ischemia (CLI) refers to a severe state of the leg in which impairment of blood flow has progressed due to arteriosclerosis obliterans (ASO) and becomes severe. Its symptoms include leg pain occurring not only during exercise but also during bed rest, and if CLI progresses, skin ulcers or skin necrosis (darkened skin) occurs. Endovascular treatment using a catheter or bypass operations have been performed for limb salvage, but the prognosis is poor, and it is said that 30% of the patients suffer major amputation of the lower limb, and 25% die within one year of onset. Recently, gene therapy or cell therapy as new therapies to regenerate blood vessels has been attempted.
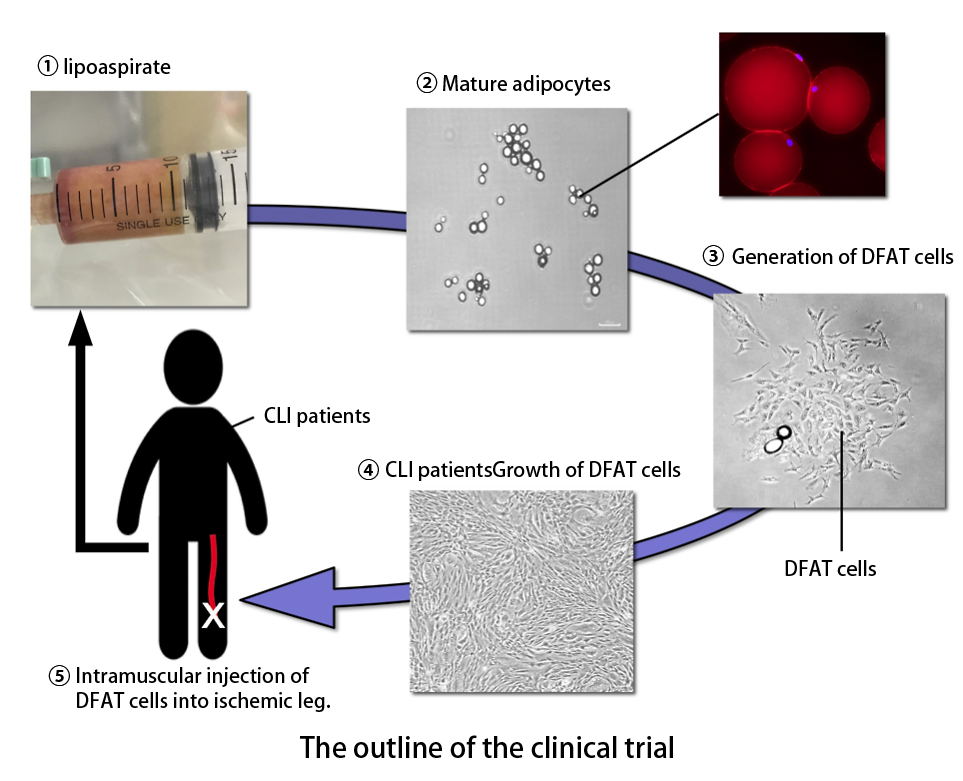
What are DFAT cells
Fat tissue, which exists abundantly in the human body, is not simply a mass of fat but is comprised of a collection of cells called adipocytes. These cells have a balloon-like shape because they have a large pouch that stores up oil droplets, and they play a role as a storehouse of energy in the body. Our research group at Nihon University independently devised a method to remove the adipocytes from adipose tissue and transform them into immature cells by culturing them using a method called "ceiling culture". DFAT cells are cells obtained via this original culture, which can be freely increased and also differentiated into cells such as those of bone, cartilage, and blood vessel. In addition, various kinds of humoral factors that are secreted from the cells show effects such as improving blood flow and early curing of wounds by forming new blood vessels. DFAT cells are characterized by the ability to produce cells that show the stable effect of treatment from small amounts of adipose tissue, without being influenced by the age and underlying diseases of patients. Therefore, it is thought that a simple and highly useful cell therapy can be conducted with fewer burdens placed on patients.
Profile of the clinical study
The aim of this clinical study is to confirm the safety and efficacy of a new therapy in which DFAT cells obtained from the cultured fat cells of patients themselves are injected into their leg muscles with poor blood flow. Subjects are patients with CLI caused by ASO. First, approximately 10 mL of subcutaneous adipose tissue is aspirated and collected from a patient under local anesthesia. The collected adipose tissue is then transferred to the research center of Nihon University School of Medicine, cultured aseptically, and the DFAT cells are prepared. The production of DFAT cells takes about five weeks. After preparing the cell count necessary for transplantation, we transplant (inject) the patient’s cells into leg muscles of the limb with poor blood flow. Hospitalization is necessary at least for 5 to 7 days after transplantation. After that, we observe and examine the patient regularly in the outpatient department over 52 weeks to evaluate the safety and efficacy of the treatment. Once the safety and efficacy of this new therapy is demonstrated, it will be of great help in the treatment of patients who suffer from CLI in the future.